|
 Example of treatment of a mixture of
pollutants in industry
Example of treatment of a mixture of
pollutants in industry
This
innovative "One-pot" process consists in capturing gaseous
pollutants in a physicochemical treatment whose liquid
effluents are subsequently digested by the aerobic bio-purification
process in the treatment plant.
(A)
Simultaneous
process of absorption (capture) and organic chemical
modification of Volatile Organic Compounds (organic
and inorganic).
This operation is carried out in a single operation
on a collection installation by physico-chemical washing.
(B)
The final destruction of the capture
products after the simultaneous process of absorption
and chemical modification
This ultimate operation (B) is carried out in a biological
treatment plant.
The organic compounds present and formed During the
condensation reaction are digested by the process of
natural aerobic bio-purification of the purification
plant.
The
originality of the process resides firstly in the choice
of the reagent which combines with the pollutants to
be treated and secondly in the final natural destruction
in the wastewater treatment plant that does not generate
any new gaseous pollution.
 The example below describes the
treatment of a mixture of pollutants in a single operation
The example below describes the
treatment of a mixture of pollutants in a single operation
A
gas scrubbing column joins the vents of three "pilot"
reactors in chemical industry:
- Reactor
1 - Synthesis of an Acid Chloride
-
Reactor 2 - Synthesis of methyl ethyl sulfide
- Reactor
3 - Decarboxylation of an Acid
|
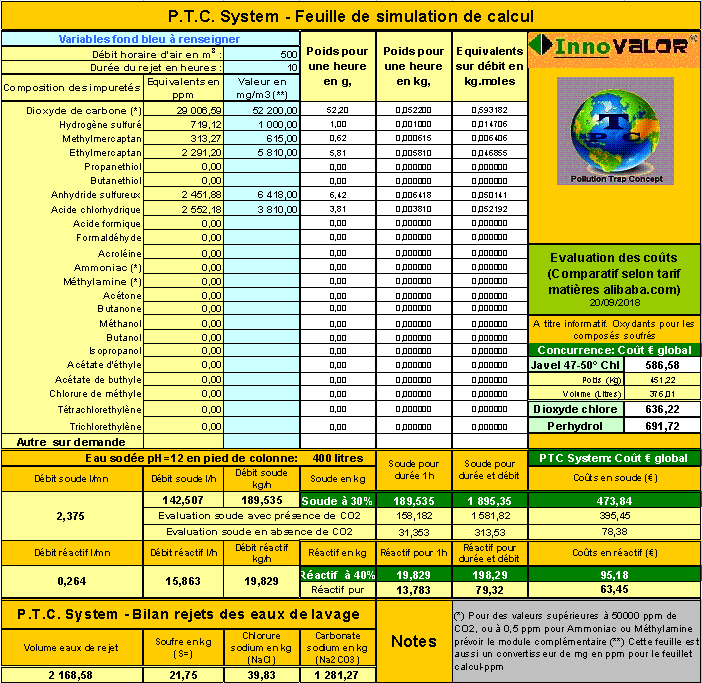
The
gaseous and volatile pollutants are composed of:
- SO2
(sulfur dioxide) 6,418 mg/m3
- HCl
(hydrochloric acid) 3 810 mg/m3
- Traces
of dichlorethane (solvent)
- Traces
of SOCl2 (thionyl chloride)
- CH3-SH
(methylmercaptan) 615 mg/m3
- C2H5-SH
(ethylmercaptan) 5 810 mg/m3
- H2S
(hydrogen sulfide) 4 000 mg/m3
- CO2
(carbon dioxide) 52 200 mg/m3
- Eventually
traces of alcohools
The
base and reagent quantities are calculated on
the worksheet
of flows and loads.
 Equipment used:
Equipment used:
-
Absorption column 3000 liters (column + tank),
trays packing, demister outgoing air.
-
Fluid circulation pump adjustable from 0 to
25 m3/h.
- Adjustable
pollutant effluent gas supply valve from 0 to
1500 m3/h.H.
 Operating load for a batch
operation:
Operating load for a batch
operation:
- Water:
400 L.
- 30%
soda: 2,010 kg
- 40%
reactive: 475 kg or 190 kg pure
|
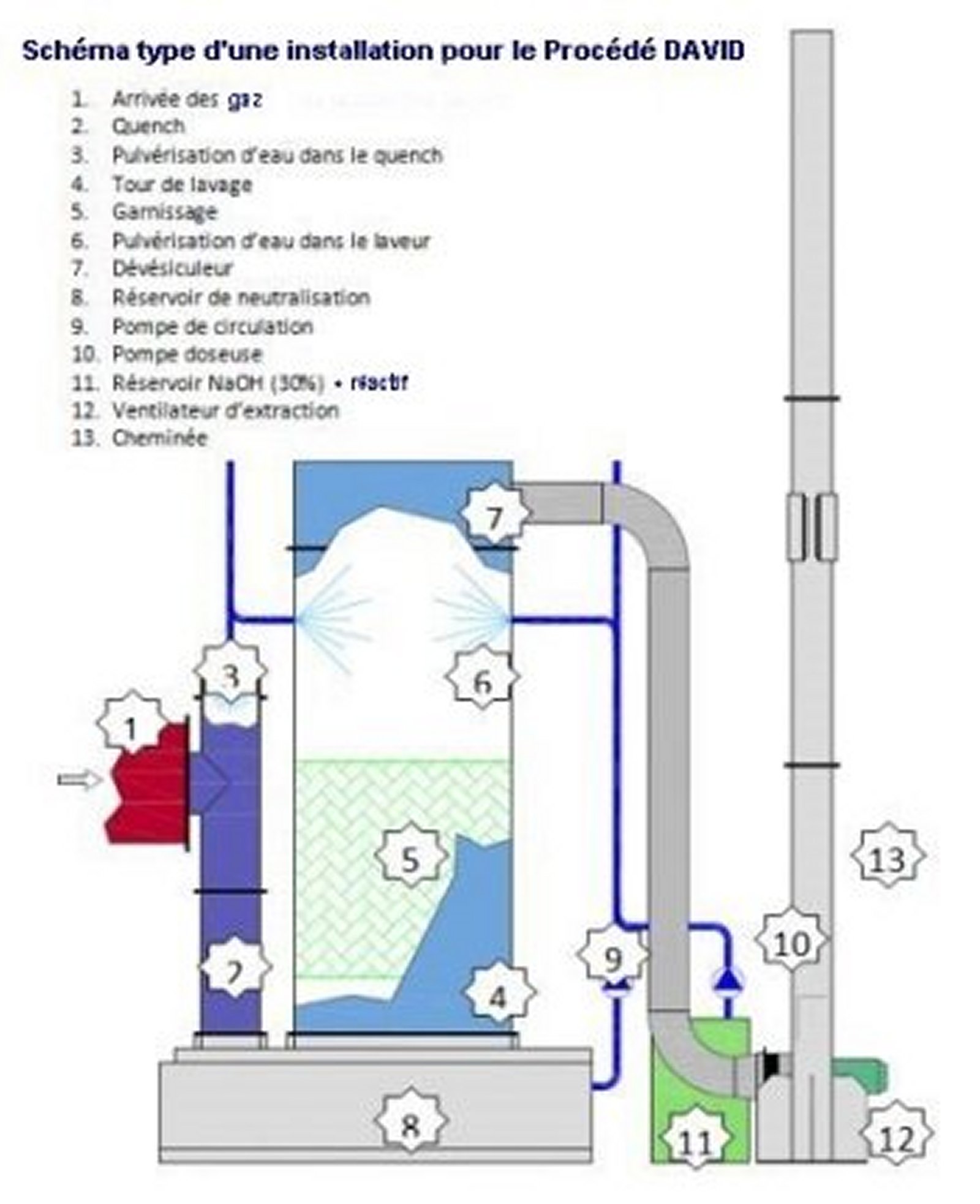
Standard
equipment
|
 Operating mode:
Operating mode:
In
the suction tank is charged the additional water determined
in the worksheet corresponding to 20 volumes of the
pure reagent and then in order the calculated amounts
of alkaline solution of potassium hydroxide, then the
40% solution of the reagent.
The reaction medium displays a pH value> 11.
The circulation pump is activated then the gas flow
valve is gradually released and controlled at the desired
flow rate.
The end of the reaction is determined and controlled
by pH <9.
The operations took place over a period of 10 hours
with a gas flow rate of 500 m3/h.
The end of the reaction is controlled by pH-metry (about
9-10), and the absence of H2S and mercaptans checked
with lead acetate paper and pH paper.
The
clear, colorless and odorless reaction medium is then
discharged to the plant's self-neutralization pit before
being discharged to the network to be subjected to the
aerobic bio-purification process in the treatment plant.
 Results on the unfiltered reaction
medium before rejection to the biological station of
the site
Results on the unfiltered reaction
medium before rejection to the biological station of
the site
- pH
= 10.3;
- temperature
35 ° C
- COD:
298 mg/L
-
BOD5: 695 mg/L
In
this test, it is clear that for the destruction of sulfur
compounds, treatment with bleach would return 12 times
more expensive than treatment with the PTC system.
ClO2 or H2O2 treatments are even more expensive.
- This
example of chemistry treatment includes:
- Complete
elimination of sulfur compounds.
- Decarbonation
(complete elimination of CO2).
- Complete
elimination of hydrochloric acid.
- Complete
removal of traces of thionyl chloride.
- The
total elimination of the solvents present in the state
of traces.
 Cost of treatment 627 €
whose:
Cost of treatment 627 €
whose:
- Soda:
503 €
- Reactive:
228 €
A
similar competitor treatment with bleach would have
cost € 1,030
For CO2 alone 1,582 liters of soda were consumed 30%
for € 395
 The
flow calculation sheet to determine the quantities of
reagents:
The
flow calculation sheet to determine the quantities of
reagents:
 See
also the examples of the DAVID Process - Odors
See
also the examples of the DAVID Process - Odors
|